








How does corrosion work?





We all know about corrosion. Materials, usually metals, are attacked by their surroundings and they rust. Often moist air is the cause.

Apart from the very few noble metals like gold, metals are unstable. You have to put a lot of energy in to extract a metal from its ore. So it's relatively easy for nature to convert metals back to a more stable form, like an oxide, sulphide or hydroxide, by a chemical (or electrochemical) reaction. What's not so obvious is that there are different kinds of corrosion, and materials can be affected in different ways.
If steels rust, that removes metal and adds oxide. The oxide is weak and loss of metal reduces the strength of the component and can lead to failure. We try to stop that by coating the metal so that the environment is kept out, for example, by painting.


Another way is to add alloying elements which protect the metal. So we add chromium to steel to make stainless steel. The chromium forms an adherent oxide on the surface which stops the steel corroding. Even if the surface is scratched, the oxide re-

Near the sea, there's a lot of salt in the air and this makes the air more aggressive and corrosion is much greater. If the steel is actually immersed, then the salt water allows electric currents to be set up between the different metals, leading to greater corrosion still (galvanic corrosion).

This can be reduced by deliberately attaching a metal more prone to corrosion so that it corrodes in preference to the steel -
For this to work, there must be good electrical contact between the two metals, and their surroundings must be conductive too -

Galvanizing, or coating with zinc usually by hot dipping, works in a similar way.


It's not just air and water that can lead to corrosion. Microbes such as bacteria can produce acids or other chemicals which can attack materials. Sewers are vulnerable to biodeterioration -
Even glass can corrode. Although it's very water-

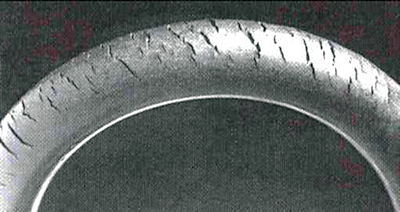
If a stress is acting on a material, even if the surrroundings are only mildly corrosive, we can get 'stress corrosion cracking'. This occurs with plastics and rubber too. Cracks in rubber due to ozone are shown on the right.
2 Aug 2016