








15 dec 2016


How does steel tempering work?

Recently, I came across an article describing the origins of the sewing needle and it’s manufacture through the ages. The article mentioned the tempering of steel needles but didn’t say what tempering was, nor why it was necessary. I felt I needed to correct this deficiency!

The article on The Humble Needle can be found HERE.


And below is an explanation of TEMPERING:

While in the past ‘tempering’ was very much an art form, later understanding made it a precise science. Details, simplified for -
Steel is iron containing carbon. Carbon modifies the structure of the iron. The equilibrium microstructure at room temperature is different from those at different higher temperatures. That's because the presence of carbon alters the crystal structure of the iron. Also, different structures (phases) are stable at different temperatures. As if this weren't complicated enough, the nature of the phases depends on the amount of carbon present! So you can see that the steel structure can be quite different, depending on the temperature and the amount of carbon in the steel.
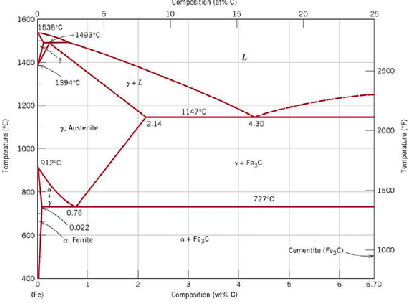

Note the term 'equilibrium'. This means the structure that exists at a given temperature if the steel is held at that temperature long enough so that all changes to the microstructure have taken place and no further change will occur -
You can make steel harder by heat-


Metals deform by various micromechanisms and these involve movement of atoms. Any stresses and/or different phases in the structure can make this more difficult. The metal is harder. In steel, forcing the high temperature structure to exist at room temperature makes the steel very brittle. That limits the usefulness of the steel. You can reduce the brittleness while maintaining a lot of the strength by carefully reheating, for a short time, the quenched structure to a temperature well below that prior to quenching. This is 'tempering'. It allows the structure to change slightly towards the equilibrium microstructure, while never actually getting there, thereby reducing internal stresses and softening the brittle microstructure. The steel still remains far from the equilibrium structure and so remains stronger, while the undesirable brittleness is removed.

That's a simple summary. All of the above can be quantified in detail, all the different steel phases characterised -
Tempering is thus no longer an art. And so for example 'Toledo' steel, much prized in the past as the perfect material for making swords, no longer requires a young goat, of particular parentage, colour, and age, to be available for quenching a white hot steel blade . . . .

